Chapter 1 Review
Key Terms
charging by induction
process by which an electrically charged object brought near a neutral object creates a charge separation in that object
conduction electron
electron that is free to move away from its atomic orbit
conductor
material that allows electrons to move separately from their atomic orbits; object with properties that allow charges to move about freely within it
continuous charge distribution
total source charge composed of so large a number of elementary charges that it must be treated as continuous, rather than discrete
coulomb
SI unit of electric charge
Coulomb force
another term for the electrostatic force
Coulomb’s law
mathematical equation calculating the electrostatic force vector between two charged particles
dipole
two equal and opposite charges that are fixed close to each other
dipole moment
property of a dipole; it characterizes the combination of distance between the opposite charges, and the magnitude of the charges
electric charge
physical property of an object that causes it to be attracted toward or repelled from another charged object; each charged object generates and is influenced by a force called an electric force
electric field
physical phenomenon created by a charge; it “transmits” a force between a two charges
electric force
noncontact force observed between electrically charged objects
electron
particle surrounding the nucleus of an atom and carrying the smallest unit of negative charge
electrostatic attraction
phenomenon of two objects with opposite charges attracting each other
electrostatic force
amount and direction of attraction or repulsion between two charged bodies; the assumption is that the source charges remain motionless
electrostatic repulsion
phenomenon of two objects with like charges repelling each other
electrostatics
study of charged objects which are not in motion
field line
smooth, usually curved line that indicates the direction of the electric field
field line density
number of field lines per square meter passing through an imaginary area; its purpose is to indicate the field strength at different points in space
induced dipole
typically an atom, or a spherically symmetric molecule; a dipole created due to opposite forces displacing the positive and negative charges
infinite plane
flat sheet in which the dimensions making up the area are much, much greater than its thickness, and also much, much greater than the distance at which the field is to be calculated; its field is constant
infinite straight wire
straight wire whose length is much, much greater than either of its other dimensions, and also much, much greater than the distance at which the field is to be calculated
insulator
material that holds electrons securely within their atomic orbits
ion
atom or molecule with more or fewer electrons than protons
law of conservation of charge
net electric charge of a closed system is constant
linear charge density
amount of charge in an element of a charge distribution that is essentially one-dimensional (the width and height are much, much smaller than its length); its units are

neutron
neutral particle in the nucleus of an atom, with (nearly) the same mass as a proton
permanent dipole
typically a molecule; a dipole created by the arrangement of the charged particles from which the dipole is created
permittivity of vacuum
also called the permittivity of free space, and constant describing the strength of the electric force in a vacuum
polarization
slight shifting of positive and negative charges to opposite sides of an object
principle of superposition
useful fact that we can simply add up all of the forces due to charges acting on an object
proton
particle in the nucleus of an atom and carrying a positive charge equal in magnitude to the amount of negative charge carried by an electron
static electricity
buildup of electric charge on the surface of an object; the arrangement of the charge remains constant (“static”)
superposition
concept that states that the net electric field of multiple source charges is the vector sum of the field of each source charge calculated individually
surface charge density
amount of charge in an element of a two-dimensional charge distribution (the thickness is small); its units are

volume charge density
amount of charge in an element of a three-dimensional charge distribution; its units are

Key Equations
| Coulomb’s law |  |
| Superposition of electric forces |  |
| Electric force due to an electric field |  |
Electric field at point  |  |
| Field of an infinite wire |  |
| Field of an infinite plane |  |
| Dipole moment |  |
Torque on dipole in external  |  |
Summary
Electric Charge
- There are only two types of charge, which we call positive and negative. Like charges repel, unlike charges attract, and the force between charges decreases with the square of the distance.
- The vast majority of positive charge in nature is carried by protons, whereas the vast majority of negative charge is carried by electrons. The electric charge of one electron is equal in magnitude and opposite in sign to the charge of one proton.
- An ion is an atom or molecule that has nonzero total charge due to having unequal numbers of electrons and protons.
- The SI unit for charge is the coulomb (
), with protons and electrons having charges of opposite sign but equal magnitude; the magnitude of this basic charge is
- Both positive and negative charges exist in neutral objects and can be separated by bringing the two objects into physical contact; rubbing the objects together can remove electrons from the bonds in one object and place them on the other object, increasing the charge separation.
- For macroscopic objects, negatively charged means an excess of electrons and positively charged means a depletion of electrons.
- The law of conservation of charge states that the net charge of a closed system is constant.
Conductors, Insulators, and Charging by Induction
- A conductor is a substance that allows charge to flow freely through its atomic structure.
- An insulator holds charge fixed in place.
- Polarization is the separation of positive and negative charges in a neutral object. Polarized objects have their positive and negative charges concentrated in different areas, giving them a charge distribution.
Coulomb’s Law
- Coulomb’s law gives the magnitude of the force between point charges. It is
where
and
are two point charges separated by a distance
. This Coulomb force is extremely basic, since most charges are due to point-like particles. It is responsible for all electrostatic effects and underlies most macroscopic forces.
Electric Field
- The electric field is an alteration of space caused by the presence of an electric charge. The electric field mediates the electric force between a source charge and a test charge.
- The electric field, like the electric force, obeys the superposition principle
- The field is a vector; by definition, it points away from positive charges and toward negative charges.
Calculating Electric Fields of Charge Distributions
- A very large number of charges can be treated as a continuous charge distribution, where the calculation of the field requires integration. Common cases are:
- one-dimensional (like a wire); uses a line charge density
- two-dimensional (metal plate); uses surface charge density
- three-dimensional (metal sphere); uses volume charge density
- one-dimensional (like a wire); uses a line charge density
- The “source charge” is a differential amount of charge
. Calculating
Electric Field Lines
- Electric field diagrams assist in visualizing the field of a source charge.
- The magnitude of the field is proportional to the field line density.
- Field vectors are everywhere tangent to field lines.
Electric Dipoles
- If a permanent dipole is placed in an external electric field, it results in a torque that aligns it with the external field.
- If a nonpolar atom (or molecule) is placed in an external field, it gains an induced dipole that is aligned with the external field.
- The net field is the vector sum of the external field plus the field of the dipole (physical or induced).
- The strength of the polarization is described by the dipole moment of the dipole,
.
Answers to Check Your Understanding
1.1 The force would point outward.
1.2 The net force would point

below the

-axis.
1.3

1.4 We will no longer be able to take advantage of symmetry. Instead, we will need to calculate each of the two components of the electric field with their own integral.
1.5 The point charge would be

where

and

are the sides of the rectangle but otherwise identical.
1.6 The electric field would be zero in between, and have magnitude

everywhere else.
Conceptual Questions
Electric Charge
1. There are very large numbers of charged particles in most objects. Why, then, don’t most objects exhibit static electricity?
2. Why do most objects tend to contain nearly equal numbers of positive and negative charges?
3. A positively charged rod attracts a small piece of cork. (a) Can we conclude that the cork is negatively charged? (b) The rod repels another small piece of cork. Can we conclude that this piece is positively charged?
4. Two bodies attract each other electrically. Do they both have to be charged? Answer the same question if the bodies repel one another.
5. How would you determine whether the charge on a particular rod is positive or negative?
Conductors, Insulators, and Charging by Induction
6. An eccentric inventor attempts to levitate a cork ball by wrapping it with foil and placing a large negative charge on the ball and then putting a large positive charge on the ceiling of his workshop. Instead, while attempting to place a large negative charge on the ball, the foil flies off. Explain.
7. When a glass rod is rubbed with silk, it becomes positive and the silk becomes negative—yet both attract dust. Does the dust have a third type of charge that is attracted to both positive and negative? Explain.
8. Why does a car always attract dust right after it is polished? (Note that car wax and car tires are insulators.)
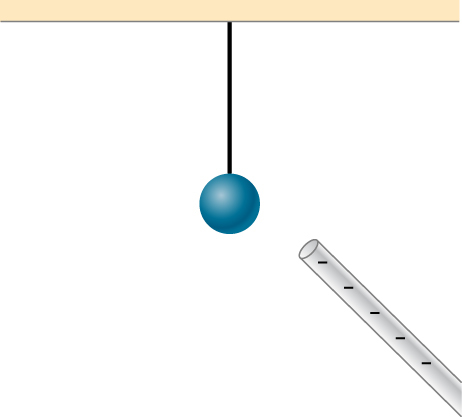
9. Does the uncharged conductor shown below experience a net electric force?
10. While walking on a rug, a person frequently becomes charged because of the rubbing between his shoes and the rug. This charge then causes a spark and a slight shock when the person gets close to a metal object. Why are these shocks so much more common on a dry day?
11. Compare charging by conduction to charging by induction.
12. Small pieces of tissue are attracted to a charged comb. Soon after sticking to the comb, the pieces of tissue are repelled from it. Explain.
13. Trucks that carry gasoline often have chains dangling from their undercarriages and brushing the ground. Why?
14. Why do electrostatic experiments work so poorly in humid weather?
15. Why do some clothes cling together after being removed from the clothes dryer? Does this happen if they’re still damp?
16. Can induction be used to produce charge on an insulator?
17. Suppose someone tells you that rubbing quartz with cotton cloth produces a third kind of charge on the quartz. Describe what you might do to test this claim.
18. A handheld copper rod does not acquire a charge when you rub it with a cloth. Explain why.
19. Suppose you place a charge

near a large metal plate. (a) If

is attracted to the plate, is the plate necessarily charged? (b) If

is repelled by the plate, is the plate necessarily charged?
Coulomb’s Law
20. Would defining the charge on an electron to be positive have any effect on Coulomb’s law?
21. An atomic nucleus contains positively charged protons and uncharged neutrons. Since nuclei do stay together, what must we conclude about the forces between these nuclear particles?
22. Is the force between two fixed charges influenced by the presence of other charges?
Electric Field
23. When measuring an electric field, could we use a negative rather than a positive test charge?
24. During fair weather, the electric field due to the net charge on Earth points downward. Is Earth charged positively or negatively?
25. If the electric field at a point on the line between two charges is zero, what do you know about the charges?
26. Two charges lie along the

-axis. Is it true that the net electric field always vanishes at some point (other than infinity) along the

-axis?
Calculating Electric Fields of Charge Distributions
27. Give a plausible argument as to why the electric field outside an infinite charged sheet is constant.
28. Compare the electric fields of an infinite sheet of charge, an infinite, charged conducting plate, and infinite, oppositely charged parallel plates.
29. Describe the electric fields of an infinite charged plate and of two infinite, charged parallel plates in terms of the electric field of an infinite sheet of charge.
30. A negative charge is placed at the center of a ring of uniform positive charge. What is the motion (if any) of the charge? What if the charge were placed at a point on the axis of the ring other than the center?
Electric Field Lines
31. If a point charge is released from rest in a uniform electric field, will it follow a field line? Will it do so if the electric field is not uniform?
32. Under what conditions, if any, will the trajectory of a charged particle not follow a field line?
33. How would you experimentally distinguish an electric field from a gravitational field?
34. A representation of an electric field shows 10 field lines perpendicular to a square plate. How many field lines should pass perpendicularly through the plate to depict a field with twice the magnitude?
35. What is the ratio of the number of electric field lines leaving a charge 10

and a charge

?
Electric Dipoles
36. What are the stable orientation(s) for a dipole in an external electric field? What happens if the dipole is slightly perturbed from these orientations?
Problems
Electric Charge
37. Common static electricity involves charges ranging from nanocoulombs to microcoulombs. (a) How many electrons are needed to form a charge of

? (b) How many electrons must be removed from a neutral object to leave a net charge of

?
38. If

electrons move through a pocket calculator during a full day’s operation, how many coulombs of charge moved through it?
39. To start a car engine, the car battery moves

electrons through the starter motor. How many coulombs of charge were moved?
40. A certain lightning bolt moves

of charge. How many fundamental units of charge is this?
41. A

–

copper penny is given a charge of

. (a) How many excess electrons are on the penny? (b) By what percent do the excess electrons change the mass of the penny?
42. A

–

copper penny is given a charge of

. (a) How many electrons are removed from the penny? (b) If no more than one electron is removed from an atom, what percent of the atoms are ionized by this charging process?
Conductors, Insulators, and Charging by Induction
43. Suppose a speck of dust in an electrostatic precipitator has

protons in it and has a net charge of

(a very large charge for a small speck). How many electrons does it have?
44. An amoeba has

protons and a net charge of

. (a) How many fewer electrons are there than protons? (b) If you paired them up, what fraction of the protons would have no electrons?
45. A

–

ball of copper has a net charge of

. What fraction of the copper’s electrons has been removed? (Each copper atom has

protons, and copper has an atomic mass of

.)
46. What net charge would you place on a

–

piece of sulfur if you put an extra electron on

in

of its atoms? (Sulfur has an atomic mass of

.)
47. How many coulombs of positive charge are there in

of plutonium, given its atomic mass is

and that each plutonium atom has

protons?
Coulomb’s Law
48. Two point particles with charges

and

are held in place by

–

forces on each charge in appropriate directions. (a) Draw a free-body diagram for each particle. (b) Find the distance between the charges.
49. Two charges

and

are fixed

apart, with the second one to the right. Find the magnitude and direction of the net force on a

–

charge when placed at the following locations: (a) halfway between the two (b) half a meter to the left of the

charge (c) half a meter above the

charge in a direction perpendicular to the line joining the two fixed charges.
50. In a salt crystal, the distance between adjacent sodium and chloride ions is

. What is the force of attraction between the two singly charged ions?
51. Protons in an atomic nucleus are typically

apart. What is the electric force of repulsion between nuclear protons?
52. Suppose Earth and the Moon each carried a net negative charge

. Approximate both bodies as point masses and point charges. (a) What value of

is required to balance the gravitational attraction between Earth and the Moon? (b) Does the distance between Earth and the Moon affect your answer? Explain. (c) How many electrons would be needed to produce this charge?
53. Point charges

and

are placed

apart. What is the force on a third charge

placed midway between

and

?
54. Where must

of the preceding problem be placed so that the net force on it is zero?
55. Two small balls, each of mass

, are attached to silk threads

long, which are in turn tied to the same point on the ceiling, as shown below. When the balls are given the same charge

, the threads hang at

to the vertical, as shown below. What is the magnitude of

? What are the signs of the two charges?

56. Point charges

and

are located at

and

. What is the force of

on

?
57. The net excess charge on two small spheres (small enough to be treated as point charges) is

. Show that the force of repulsion between the spheres is greatest when each sphere has an excess charge

. Assume that the distance between the spheres is so large compared with their radii that the spheres can be treated as point charges.
58. Two small, identical conducting spheres repel each other with a force of

when they are

apart. After a conducting wire is connected between the spheres and then removed, they repel each other with a force of

. What is the original charge on each sphere?
59. A charge

is placed at the point

shown below. What is the force on

?

60. What is the net electric force on the charge located at the lower right-hand corner of the triangle shown here?

61. Two fixed particles, each of charge

, are

apart. What force do they exert on a third particle of charge

that is

from each of them?
62. The charges

,

, and

are placed at the corners of the triangle shown below. What is the force on

?

63. What is the force on the charge

at the lower-right-hand corner of the square shown here?

64. Point charges

and

are fixed at
and

. What is the force of

on

?
Electric Field
65. A particle of charge

experiences an upward force of magnitude

when it is placed in a particular point in an electric field. (a) What is the electric field at that point? (b) If a charge

is placed there, what is the force on it?
66. On a typical clear day, the atmospheric electric field points downward and has a magnitude of approximately

. Compare the gravitational and electric forces on a small dust particle of mass

that carries a single electron charge. What is the acceleration (both magnitude and direction) of the dust particle?
67. Consider an electron that is

from an alpha particle (

). (a) What is the electric field due to the alpha particle at the location of the electron? (b) What is the electric field due to the electron at the location of the alpha particle? (c) What is the electric force on the alpha particle? On the electron?
68. Each the balls shown below carries a charge

and has a mass

. The length of each thread is

, and at equilibrium, the balls are separated by an angle

. How does

vary with

and

? Show that

satisfies

.

69. What is the electric field at a point where the force on a charge

is

?
70. A proton is suspended in the air by an electric field at the surface of Earth. What is the strength of this electric field?
71. The electric field in a particular thundercloud is

. What is the acceleration of an electron in this field?
72. A small piece of cork whose mass is

is given a charge of

. What electric field is needed to place the cork in equilibrium under the combined electric and gravitational forces?
73. If the electric field is

at a distance of

from a point charge

, what is the value of

?
74. What is the electric field of a proton at the first Bohr orbit for hydrogen (

)? What is the force on the electron in that orbit?
75. (a) What is the electric field of an oxygen nucleus at a point that is

from the nucleus? (b) What is the force this electric field exerts on a second oxygen nucleus placed at that point?
76. Two point charges,

and

, are held

apart. (a) What is the electric field at a point

from the negative charge and along the line between the two charges? (b)What is the force on an electron placed at that point?
77. Point charges

and

are placed

apart. (a) What is the electric field at a point midway between them? (b) What is the force on a charge

situated there?
78. Can you arrange the two point charges

and

along the

-axis so that

at the origin?
79. Point charges

are fixed on the

-axis at

and

. What charge

must be placed at the origin so that the electric field vanishes at

,

Calculating Electric Fields of Charge Distributions
80. A thin conducting plate

on the side is given a charge of

. An electron is placed

above the centre of the plate. What is the acceleration of the electron?
81. Calculate the magnitude and direction of the electric field 2.0 m from a long wire that is charged uniformly at

.
82. Two thin conducting plates, each

on a side, are situated parallel to one another and

apart. If

electrons are moved from one plate to the other, what is the electric field between the plates?
83. The charge per unit length on the thin rod shown below is

. What is the electric field at the point

? (Hint: Solve this problem by first considering the electric field

at

due to a small segment

of the rod, which contains charge

. Then find the net field by integrating

over the length of the rod.)

84. The charge per unit length on the thin semicircular wire shown below is

. What is the electric field at the point

?

85. Two thin parallel conducting plates are placed

apart. Each plate is

on a side; one plate carries a net charge of

and the other plate carries a net charge of

What is the charge density on the inside surface of each plate? What is the electric field between the plates?
86. A thin conducing plate

on a side is given a total charge of

(a) What is the electric field

above the plate? (b) What is the force on an electron at this point? (c) Repeat these calculations for a point

above the plate. (d) When the electron moves from

to

above the plate, how much work is done on it by the electric field?
87. A total charge

is distributed uniformly along a thin, straight rod of length

(see below). What is the electric field at

? At

?

88. Charge is distributed along the entire

-axis with uniform density

. How much work does the electric field of this charge distribution do on an electron that moves along the

-axis from

to

?
89. Charge is distributed along the entire

-axis with uniform density

and along the entire

-axis with uniform density

. Calculate the resulting electric field at (a)

and (b)

90. A rod bent into the arc of a circle subtends an angle

at the centre

of the circle (see below). If the rod is charged uniformly with a total charge

, what is the electric field at

?

91. A proton moves in the electric field

. (a) What are the force on and the acceleration of the proton? (b) Do the same calculation for an electron moving in this field.
92. An electron and a proton, each starting from rest, are accelerated by the same uniform electric field of

. Determine the distance and time for each particle to acquire a kinetic energy of

.
93. A spherical water droplet of radius

carries an excess

electrons. What vertical electric field is needed to balance the gravitational force on the droplet at the surface of the earth?
94. A proton enters the uniform electric field produced by the two charged plates shown below. The magnitude of the electric field is

and the speed of the proton when it enters is

What distance

has the proton been deflected downward when it leaves the plates?

95. Shown below is a small sphere of mass

that carries a charge of

The sphere is attached to one end of a very thin silk string

long. The other end of the string is attached to a large vertical conducting plate that has a charge density of

What is the angle that the string makes with the vertical?

96. Two infinite rods, each carrying a uniform charge density

are parallel to one another and perpendicular to the plane of the page. (See below.) What is the electrical field at

? At

?

97. Positive charge is distributed with a uniform density

along the positive

-axis from

to

, along the positive

-axis from

to

, and along a

arc of a circle of radius

as shown below. What is the electric field at

?

98. From a distance of

, a proton is projected with a speed of

directly at a large, positively charged plate whose charge density is

. (See below.) (a) Does the proton reach the plate? (b) If not, how far from the plate does it turn around?

99. A particle of mass

and charge

moves along a straight line away from a fixed particle of charge

. When the distance between the two particles is


is moving with a speed

. (a) Use the work-energy theorem to calculate the maximum separation of the charges. (b) What do you have to assume about v0v0 to make this calculation? (c) What is the minimum value of

such that

escapes from

?
Electric Field Lines
100. Which of the following electric field lines are incorrect for point charges? Explain why.

101. In this exercise, you will practice drawing electric field lines. Make sure you represent both the magnitude and direction of the electric field adequately. Note that the number of lines into or out of charges is proportional to the charges. (a) Draw the electric field lines map for two charges

and
situated

from each other. (b) Draw the electric field lines map for two charges

and

situated

from each other. (c) Draw the electric field lines map for two charges

and

situated

from each other.
102. Draw the electric field for a system of three particles of charges


and

fixed at the corners of an equilateral triangle of side

.
103. Two charges of equal magnitude but opposite sign make up an electric dipole. A quadrupole consists of two electric dipoles are placed anti-parallel at two edges of a square as shown.

Draw the electric field of the charge distribution.
104. Suppose the electric field of an isolated point charge decreased with distance as

rather than as

. Show that it is then impossible to draw continuous field lines so that their number per unit area is proportional to

.
Electric Dipoles
105. Consider the equal and opposite charges shown below. (a) Show that at all points on the

-axis for which

,

. (b) Show that at all points on the

-axis for which

,

.

106. (a) What is the dipole moment of the configuration shown above? If

, (b) what is the torque on this dipole with an electric field of

? (c) What is the torque on this dipole with an electric field of

? (d) What is the torque on this dipole with an electric field of

?
107. A water molecule consists of two hydrogen atoms bonded with one oxygen atom. The bond angle between the two hydrogen atoms is

(see below). Calculate the net dipole moment of a water molecule that is placed in a uniform, horizontal electric field of magnitude

. (You are missing some information for solving this problem; you will need to determine what information you need, and look it up.)

Additional Problems
108. Point charges

and

are located at

and

. What is the force of

on

?
109. What is the force on the

–

charge shown below?

110. What is the force on the

–

charge placed at the centre of the square shown below?

111. Four charged particles are positioned at the corners of a parallelogram as shown below. If

and

what is the net force on

?

112. A charge

is fixed at the origin and a second charge

moves along the

-axis, as shown below. How much work is done on

by the electric force when

moves from

to


113. A charge

is released from rest when it is

from a fixed charge

What is the kinetic energy of

when it is

from

?
114. What is the electric field at the midpoint

of the hypotenuse of the triangle shown below?

115. Find the electric field at

for the charge configurations shown below.

116. (a) What is the electric field at the lower-right-hand corner of the square shown below? (b) What is the force on a charge

placed at that point?

117. Point charges are placed at the four corners of a rectangle as shown below:



and

What is the electric field at

?

118. Three charges are positioned at the corners of a parallelogram as shown below. (a) If

what is the electric field at the unoccupied corner? (b) What is the force on a

–

charge placed at this corner?

119. A positive charge

is released from rest at the origin of a rectangular coordinate system and moves under the influence of the electric field

What is the kinetic energy of

when it passes through

?
120. A particle of charge

and mass

is placed at the centre of a uniformly charged ring of total charge

and radius

. The particle is displaced a small distance along the axis perpendicular to the plane of the ring and released. Assuming that the particle is constrained to move along the axis, show that the particle oscillates in simple harmonic motion with a frequency

121. Charge is distributed uniformly along the entire

-axis with a density

and along the positive

-axis from

to

with a density

. What is the force between the two distributions?
122. The circular arc shown below carries a charge per unit length

where

is measured from the

-axis. What is the electric field at the origin?

123. Calculate the electric field due to a uniformly charged rod of length

, aligned with the

-axis with one end at the origin; at a point

on the

-axis.
124. The charge per unit length on the thin rod shown below is

What is the electric force on the point charge

? Solve this problem by first considering the electric force

on

due to a small segment

of the rod, which contains charge

Then, find the net force by integrating

over the length of the rod.

125. The charge per unit length on the thin rod shown here is

What is the electric force on the point charge

? (See the preceding problem.)

126. The charge per unit length on the thin semicircular wire shown below is

What is the electric force on the point charge

? (See the preceding problems.)

Candela Citations
CC licensed content, Specific attribution
- Download for free at http://cnx.org/contents/7a0f97...; Retrieved from: http://cnx.org/contents/7a0f97...; License: CC BY: Attribution
Introduction to Electricity, Magnetism, and Circuits by Daryl Janzen is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted.
Explore CircuitBread

Get the latest tools and tutorials, fresh from the toaster.











