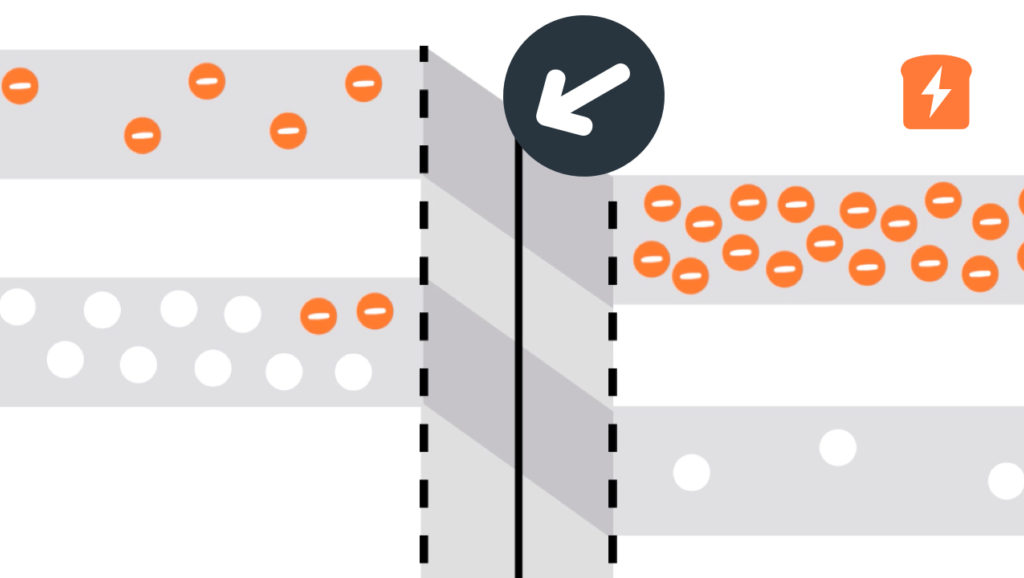
Band gap
With semiconductors, how much energy electrons have is of the utmost importance. When moving from the valence band to the conduction band, electrons need to gain enough energy to do so. The amount of energy needed is the gap between the two bands - hence “band gap”! It’s nice when things make sense…
The difference in energy between energy levels in an atom.
Electronic Devices : Conventional Current Version, 9th Edition by Thomas L. Floyd
In solid-state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap
in chemistry. If the valence band is completely full and the conduction
band is completely empty, then electrons cannot move in the solid;
however, if some electrons transfer from the valence to the conduction
band, then current can flow (see carrier generation and recombination). Therefore, the band gap is a major factor determining the electrical conductivity of a solid. Substances with large band gaps are generally insulators, those with smaller band gaps are semiconductors, while conductors either have very small band gaps or none, because the valence and conduction bands overlap.